Multiple Choice
Identify the reducing agent in the following reaction. Fe2+(aq) 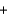 Ce4+(aq)
Ce4+(aq)  Fe3+(aq)
Fe3+(aq) 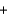 Ce3+(aq)
Ce3+(aq)
A) Fe2+(aq)
B) Fe3+(aq)
C) Ce3+(aq)
D) Ce4+(aq)
E) This is not an example of an oxidation-reduction reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which contains more solute particles: a 0.10
Q9: Draw pictures that represent: (a) a strong
Q10: Sodium fluoride is added to drinking water
Q11: In which one of the following compounds
Q12: Why is an aqueous solution of table
Q14: If one regular antacid tablet contains 500
Q15: Identify which of the following compounds are
Q16: Write the net ionic equation for the
Q17: Which one of the following statements is
Q18: In the dilution of 10.0 mL of