Essay
Identify the species that is oxidized and reduced in the following oxidation-reduction reaction.
Fe2O3(s) 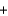 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 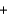 +3CO2(g)
+3CO2(g)
Correct Answer:

Verified
Oxidized: ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
Oxidized: ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q138: Based on the activity series shown below,
Q139: If 120 g of NaOH were used
Q140: Water-soluble toxic chromium compounds are waste products
Q141: How many moles of ammonium hydroxide are
Q142: Which picture best represents an atomic-level view
Q143: How many liters of 0.200 M NaOH
Q144: Hard water contains Mg<sup>2+</sup> and Ca<sup>2+</sup> ions
Q145: The amount of blood in the human
Q146: In which one of the following compounds
Q148: In the following reaction, H<sub>2</sub>O _ CH<sub>3</sub>COOH(aq)