Multiple Choice
Copper sulfate is a blue solid that is used to control algae growth. Solutions of copper sulfate that come in contact with the surface of galvanized (zinc-plated) steel pails undergo the following reaction that forms copper metal on the zinc surface. How many grams of zinc would react with 454 g (1 lb) of copper sulfate (160 g/mol) ? CuSO4(aq) 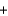 Zn(s)
Zn(s)  Cu(s)
Cu(s) 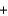 ZnSO4(aq)
ZnSO4(aq)
A) 467 g
B) 186 g
C) 93 g
D) 234 g
E) 454 g
Correct Answer:

Verified
Correct Answer:
Verified
Q52: At the Tesla automotive factory, a Model
Q53: A reaction vessel contains equal masses of
Q54: Respiration is to photosynthesis as _<br>A)a lake
Q55: Which molecules shown below have the same
Q56: For which of the following compounds are
Q58: What mass of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>, 98.0
Q59: As a purchasing agent for a pharmaceutical
Q60: Which statement about the following chemical
Q61: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q62: Which statement about a balanced chemical reaction