Multiple Choice
Which element labeled A-E in the periodic table below will have an ionic charge of +2? 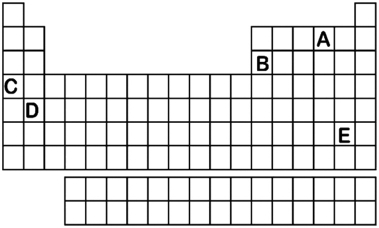
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: Which statement best describes isotopes?<br>A)They have the
Q92: What is the symbol of the ion
Q93: An unknown element is found to contain
Q94: What is the correct symbol for a
Q95: Write the complete atomic symbol with both
Q97: Name the following oxides of nitrogen in
Q98: Silicon is best described as a _<br>A)metalloid.<br>B)metal.<br>C)transition
Q99: Identify the letter of the group that
Q100: Iron is best described as a(n) _<br>A)metalloid.<br>B)transition
Q101: He is the symbol for _<br>A)hydrogen.<br>B)hafnium.<br>C)mercury.<br>D)helium.<br>E)holmium.