Multiple Choice
Starting with the expression for work 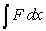 , one can change the look of the expression without changing what it represents by dividing the force F by the area it is applied over and multiplying the differential distance dx by that same area. Force divided by area is a pressure (P) , and area times the differential distance is a differential volume. Hence one can also describe work as
, one can change the look of the expression without changing what it represents by dividing the force F by the area it is applied over and multiplying the differential distance dx by that same area. Force divided by area is a pressure (P) , and area times the differential distance is a differential volume. Hence one can also describe work as 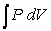 . A gas at constant temperature will change its pressure inversely to changes in volume,
. A gas at constant temperature will change its pressure inversely to changes in volume, 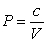 . If a sample of gas has a pressure of 1 atmosphere when its volume is 1 L, how much work does it do when it expands from 1 L to 4 L?
. If a sample of gas has a pressure of 1 atmosphere when its volume is 1 L, how much work does it do when it expands from 1 L to 4 L?
A) 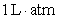
B) 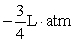
C) 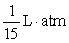
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Where is the center of mass of
Q13: Sketch the solid bounded by <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg"
Q14: Find the area between the following curves
Q15: An object is dropped from a height
Q16: Identify the integral used to determine the
Q18: A given sample of a particular polymer
Q19: The base of a solid V is
Q20: Is the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Is the
Q21: Is the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2342/.jpg" alt="Is the
Q22: Given the pdf for a particular exam