Multiple Choice
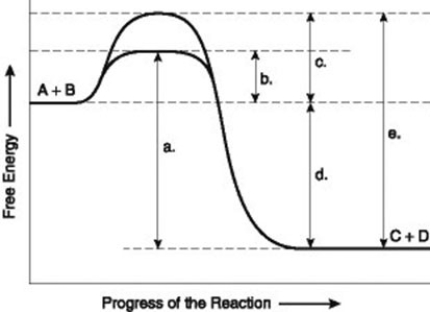
-The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describes the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
Correct Answer:

Verified
Correct Answer:
Verified
Q9: How might a change of one amino
Q17: HIV is the virus that causes AIDS.
Q26: Choose the pair of terms that correctly
Q34: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6149/.jpg" alt=" - Activity of
Q35: When chemical, transport, or mechanical work is
Q36: A decrease in entropy is associated with
Q41: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6149/.jpg" alt=" - The
Q43: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6149/.jpg" alt=" Rate of an
Q55: Which of the following statements describes a
Q62: Biological systems use free energy based on