Multiple Choice
The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose. Side A is half-filled with a solution of 2 M sucrose and 1 M glucose. Side B is half-filled with 1 M sucrose and 2 M glucose. Initially, the liquid levels on both sides are equal. 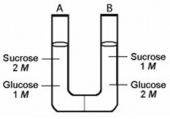 Which of the following will be true when the system illustrated above reaches equilibrium?
Which of the following will be true when the system illustrated above reaches equilibrium?
A) The concentration of sucrose on side A will be greater than the concentration of sucrose on side B.
B) The water level will be higher in side A than in side B.
C) The water levels will be unchanged.
D) The water level will be higher in side B than in side A.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: According to the fluid mosaic model of
Q24: In receptor-mediated endocytosis, receptor molecules initially project
Q29: Match the labelled component of the cell
Q31: Match the labelled component of the cell
Q31: Which of the following types of molecules
Q36: The solutions in the two arms of
Q38: Which of the following statements about diffusion
Q38: Which of the following membrane activities requires
Q39: The membranes of winter wheat are able
Q65: A patient was involved a serious accident