Multiple Choice
Refer to the following figure to answer the questions below.
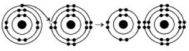
-What results from the chemical reaction in the illustration? The reactants have no charge.
A) a cation with a net charge of +1 and an anion with a net charge of +1
B) a cation with a net charge of -1 and an anion with a net charge of -1
C) a cation with a net charge of -1 and an anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of -1
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Which one of the atoms shown would
Q35: What is the difference between covalent bonds
Q36: Refer to the following figure (first three
Q37: A covalent chemical bond is one in
Q39: An atom has four electrons in its
Q41: What is the maximum number of hydrogen
Q42: The atomic number of nitrogen is 7.
Q43: We can represent atoms by listing the
Q44: If an atom has a charge of
Q44: The left to right order of elements