Essay
Instructions: For each of the compounds below tell how many signals you would expect the molecule to have in its normal, broadband decoupled 13C NMR spectra.
Number of signals: 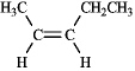
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Instructions: Refer to the mass spectrum of
Q17: Instructions: Predict the splitting patterns you would
Q18: Instructions: Match each of the following groups
Q19: Instructions: The following questions pertain to the
Q20: Which of the following compounds gives an
Q22: Which of the following compounds would produce
Q23: Circle any of the following compounds that
Q24: Instructions: Refer to the structure of 3-methylbutan-2-one
Q25: Consider the compound below: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4942/.jpg" alt="Consider
Q26: Which of the following compounds gives an