Multiple Choice
Which of the following best represents the shape of a sp3 hybrid orbital of carbon? 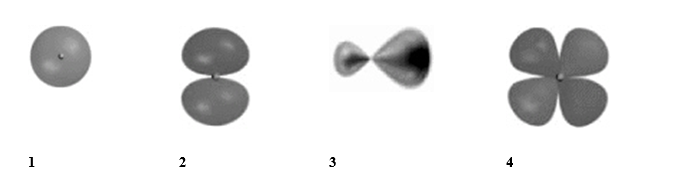
A) 1
B) 2
C) 3
D) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Convert the skeletal drawing of the pharmaceutical
Q31: Which is the strongest base (pK<sub>a</sub> values
Q32: Instructions: Consider the two structures below
Q33: Of the bonds found in <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4942/.jpg"
Q34: How many total valence electrons are represented
Q36: Identify the reactants and product in the
Q37: Instructions: Use the convention d-/d+ and the
Q38: Draw the structure for CCl<sub>2</sub>F<sub>2</sub> using solid,
Q39: Circle the Lewis bases in the group
Q40: How many nonbonding electron pairs are in