Essay
Ingestion of mercury, which is poisonous, often is treated using chelation therapy. One agent used in this therapy is dimercaprol, which is shown below. Identify the features in the structure of this molecule that make it an effective chelation agent. 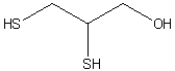
Correct Answer:

Verified
The sulfur and oxygen atoms on this liga...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q26: The pK<sub>a</sub> of hydrated iron(III) is 2.5,
Q31: Identify the Lewis acid in the
Q36: Which of the following complexes has the
Q37: What is the equilibrium concentration of
Q60: One of the following solutions is acidic.
Q66: Calculate the equilibrium concentration of Zn<sup>2+</sup>(aq)
Q83: What is the shape of the complex
Q86: In the following reaction, which species
Q89: A Lewis base is _<br>A)an electron-pair acceptor.<br>B)an
Q91: The reaction<br>Cr(NH<sub>3</sub>)<sub>6</sub><sup>3+</sup>(aq) + 3en(aq) <span class="ql-formula"