Multiple Choice
The base ionization constant Kb describes which of the following reactions for a weak base, B, in aqueous solution?
A) B + H+ 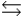 BH+
BH+
B) B + H3O+ 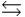 BH+ + H2O
BH+ + H2O
C) B + H2O 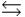 BH+ + OH-
BH+ + OH-
D) B + OH- 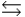 BH- + O2-
BH- + O2-
E) BH+ + OH- 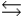 B + H2O
B + H2O
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: A solution with a pOH of 6.92
Q61: A cleaning solution has a pOH of
Q63: Acid-base indicators change color _<br>A) exactly when
Q65: Acid-base indicators need to have very intense
Q65: A phosphate buffer solution (25.00 mL sample)
Q67: When an acetic acid solution is titrated
Q78: The degree of ionization _<br>A)increases with increasing
Q81: The acidic ingredient in vinegar is acetic
Q95: A solution that contains a weak acid
Q118: The pH of a popular soft drink