Multiple Choice
In the following reaction in aqueous solution, the acid reactant is __________, and its conjugate base product is __________. CH3NH2 + HSO4- 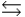 CH3NH3+ + SO42-
CH3NH3+ + SO42-
A) CH3NH2; CH3NH3+
B) CH3NH2; SO42-
C) HSO4-; CH3NH3+
D) HSO4-; SO42-
E) HSO4-; H3O+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: In the Brønsted-Lowry definition of acids and
Q31: Which one of the following statements is
Q35: Derive the Henderson-Hasselbalch equation from the acid
Q50: The pH of an aqueous sodium fluoride
Q114: Bert and Ernie were determining the pH
Q121: The solubility product for an insoluble salt
Q125: A phosphate buffer solution (25.00 mL sample)
Q131: Three acids found in foods are lactic
Q154: The most suitable acid-base indicator for a
Q174: Which one of the following salts forms