Multiple Choice
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 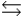 H2PO4- + H3O+ Ka
H2PO4- + H3O+ Ka
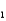
H2PO4- + H2O 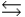 HPO42- + H3O+ Ka
HPO42- + H3O+ Ka
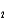 HPO42- + H2O
HPO42- + H2O 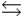 PO43- + H3O+ Ka
PO43- + H3O+ Ka
 What is the Kb expression for the base, sodium phosphate?
What is the Kb expression for the base, sodium phosphate?
A) 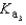
B) 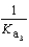
C) 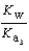
D) 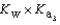
E) 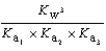
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The concentration of acetic acid in vinegar
Q27: In each of the following, the stronger
Q28: A mining operation needs to separate silver
Q29: What is the pH of a solution
Q37: Which sketch best represents the qualitative molecular
Q94: Which expression defines the autoionization constant for
Q111: Which ending to the statement is not
Q111: What is the pOH of a 0.20
Q115: Four solutions have pH values of 3,
Q171: What is the hydronium ion concentration of