Multiple Choice
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 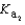 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
A) nearly 14
B) slightly above 7.00
C) 7.00
D) slightly below 7.00
E) nearly 1.00
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What are the characteristics of a pH
Q26: Calculate the pH of a solution that
Q65: Write the reaction and equilibrium constant that
Q70: Carbon dioxide in the atmosphere dissolves in
Q76: Use the following acid ionization constants to
Q77: One brand of extra-strength antacid tablets contains
Q77: At what point in the following titration
Q89: A buffer system is set up with
Q120: Which one of the following is not
Q124: Glycolic acid, which is a monoprotic acid