Multiple Choice
The following titration curve is most likely to be associated with 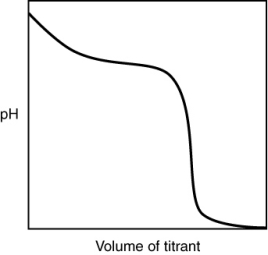
A) the titration of a strong acid with a strong base titrant.
B) the titration of a weak acid with a strong base titrant.
C) the titration of a strong base with a strong acid titrant.
D) the titration of a weak base with a strong acid titrant.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: What is the pH of a 0.25
Q21: The solubility of PbBr<sub>2</sub> is 0.427 g
Q41: A solution of sulfuric acid (H<sub>2</sub>SO<sub>4</sub>, 25.00
Q91: Which one of the following is a
Q93: Define a conjugate acid-base pair and provide
Q120: Which of the following would be the
Q132: Phenylephrine (PE, see the structure below) is
Q134: Vitamin C is a monoprotic weak acid,
Q164: Pure water at any temperature has _<br>A)
Q178: Which of the following compounds cannot be