Multiple Choice
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution. Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution. The resulting titration curves are illustrated here. Given the following possibilities, what is the sample? 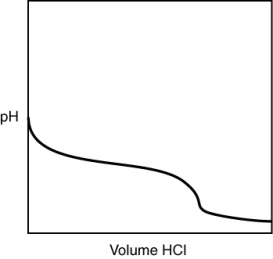
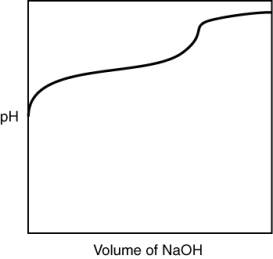
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The solubility product for an insoluble salt
Q58: A 0.100 M solution of a monoprotic
Q66: As the pH decreases, the solubility of
Q70: The solubility product for an insoluble salt
Q92: Sometimes liquid ammonia, NH<sub>3</sub>, is used as
Q99: Which one of the following salts does
Q107: Stalactites-the long, icicle-like formations that hang from
Q114: Bert and Ernie were determining the pH
Q134: What is the pH of a buffer
Q162: Halfway to the equivalence point in a