Multiple Choice
Indicate which one of the following reactions results in a negative Ssys.
A) H2O(g) H2O(s)
B) CaCO3(s) CaO(s) + CO2(g)
C) CuSO4 · 5H2O(s) CuSO4(s) + 5H2O(g)
D) 14O2(g) + 3NH4NO3(s) + C10H22( 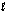 ) 3N2(g) + 17H2O(g) + 10CO2(g)
) 3N2(g) + 17H2O(g) + 10CO2(g)
E) CO2(aq) CO2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which of the following are true
Q11: Consider a closed container containing a 1
Q26: What are the signs of <span
Q42: At constant T and P, any
Q56: The standard molar entropy of lead(II) bromide
Q57: Determine <font face="symbol"></font>G<sub>rxn</sub> for C<sub>4</sub>H<sub>10</sub>( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3833/.jpg"
Q62: The following figures represent distributions of two
Q96: Benzene is a liquid under standard conditions.
Q116: The standard molar enthalpy of fusion for
Q124: Which of the following is in the