Multiple Choice
Indicate which of the following has the lowest standard molar entropy (S°) .
A) CH4(g)
B) CH3CH2OH( 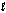 )
)
C) H2O(s)
D) Na(s)
E) He(g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: Considering the tabulated values for the thermodynamic
Q47: The standard entropy of diamond is 2.4
Q48: What is the entropy change to the
Q56: The standard molar entropy of lead(II) bromide
Q62: The equilibrium vapor pressure for benzene
Q86: Give an example of a spontaneous and
Q89: If 1 mol of ice melts at
Q123: Which of the following must be
Q125: Indicate which of the following has the
Q147: The entropy change in a system (<font