Essay
What is the overall standard free-energy change for the following two reactions in terms of G1 and G2?
A + B 2C G 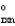 = G1
= G1
C + D E G 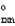 = G2
= G2
Correct Answer:

Verified
As C is an intermedi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
As C is an intermedi...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q10: The standard entropy of N<sub>2</sub>(g) is 191.5
Q14: Carbon monoxide has a very weak dipole
Q17: Hydrogen reacts with nitrogen to form ammonia
Q18: At body temperature, many proteins have a
Q19: Which, if any, of A through D
Q45: Carbon atoms can be found in a
Q56: Boltzmann derived the relationship, S = k
Q80: What types of motion does a molecule
Q122: Which of the following must be true
Q134: When you increase the volume of a