Multiple Choice
Determine the energy change for the reaction Li(s) + 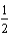 Cl2(g) LiCl(s)
Cl2(g) LiCl(s)
From the following data:
Lattice energy of LiCl = -861 kJ/mol
Energy to vaporize Li = 159 kJ/mol
Ionization energy of Li = 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: -349 kJ/mol
A) -411 kJ/mol
B) -528 kJ/mol
C) 311 kJ/mol
D) -861 kJ/mol
E) -291 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Does the temperature of the vapors in
Q20: Which statement below regarding vapor pressure is
Q32: Determine the molal concentration of a sugar
Q42: Which of the following would not be
Q44: A mixture of salt and ice is
Q45: The arrow in the diagram below indicates
Q56: Indicate which aqueous solution has the highest
Q64: What information is provided by the van
Q73: Which one of the ionic compounds below
Q99: The aroma from almonds and cherries is