Multiple Choice
Which one of the following substances would you predict to have the highest boiling point? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 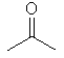
E) 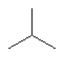
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Which of the following compounds would be
Q31: Which of the following compounds will have
Q34: The density of water decreases as it
Q43: Water contains about 42 mg of oxygen
Q46: Why does HI boil at a higher
Q49: The temperature at point b in the
Q52: The relative energies (strengths) of the intermolecular
Q53: Based on their boiling points, which of
Q66: Which of the following compounds would you
Q116: At the triple point of a substance,