Multiple Choice
Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 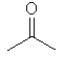
E) 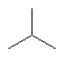
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Would water rise to the same height
Q76: Henry's law constant (mol/L · atm) for
Q87: At the point marked with a dot
Q88: The relative energies (strengths) of the intermolecular
Q89: Henry's law constant for oxygen dissolving in
Q90: The temperature at point a in the
Q91: Ion interaction energies are determined by Coulomb's
Q94: Indicate which of the following compounds will
Q95: Henry's law constant for oxygen dissolving in
Q97: Identify the dominant intermolecular interaction or interactions