Multiple Choice
Which statement concerning benzene is not correct? 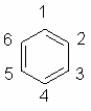
A) A second Lewis structure is needed to describe benzene.
B) All the bonds originate from p atomic orbitals.
C) Benzene does not have a dipole moment.
D) The carbon atomic orbitals are sp2 hybridized.
E) Benzene has both long and short carbon-carbon bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Which of the following ions is linear?<br>A)
Q58: Which of the following is a planar
Q70: Which of the following compounds has the
Q83: Which one of the following statements
Q85: Use energy levels of diatomic molecules derived
Q101: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A)linear<br>B)bent<br>C)trigonal bipyramidal<br>D)tetrahedral<br>E)trigonal
Q114: Which of the following molecules has a
Q120: What is the hybridization of the central
Q126: Which of the following compounds has the
Q156: Which bond angle is the smallest?<br>A)H-C-H in