Essay
Use the relative energies of the molecular orbitals given below, which are derived from the n = 5 atomic orbitals, to determine the bond orders in I2 , 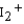 , and
, and 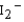 , and identify the species that is predicted to be the least stable. The valence molecular orbitals in order of increasing energy are < * < < < * <*.
, and identify the species that is predicted to be the least stable. The valence molecular orbitals in order of increasing energy are < * < < < * <*.
Correct Answer:

Verified
The valence electron configuration of 
...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q22: Which of the following compounds is the
Q25: Broccoli, cabbage, and kale contain compounds that
Q26: For the series methane, ammonia, and water,
Q85: Use energy levels of diatomic molecules derived
Q86: What are the hybridizations of the carbon
Q120: What is the hybridization of the central
Q126: Which of the following compounds has the
Q140: The local molecular geometry and the hybridization
Q154: Identify the polar molecule.<br>A)CF<sub>4</sub><br>B)SiH<sub>4</sub><br>C)CHCl<sub>3</sub><br>D)CS<sub>2</sub><br>E)CO<sub>2</sub>
Q156: Which bond angle is the smallest?<br>A)H-C-H in