Multiple Choice
Identify the acid in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 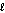 )
)
A) PbCO3(s)
B) CO2(g)
C) PbSO4(s)
D) H2O( 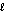 )
)
E) H2SO4(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Diluting 1.0 mL of a 1.0 M
Q35: Define the terms oxidizing agent and reducing
Q56: Tube worms that survive near geothermal vents
Q66: Some microorganisms living under anaerobic conditions extract
Q71: Water-soluble toxic chromium compounds are waste products
Q85: Identify the solution below that will conduct
Q98: A food chemist determined the concentration of
Q122: Solutions of hydrochloric acid, sulfuric acid, and
Q134: Chalk contains calcium carbonate. What would be
Q143: How many liters of 0.200 M NaOH