Multiple Choice
Identify the base in the following acid-base reaction. PbCO3(s) + H2SO4(aq) PbSO4(s) + CO2(g) + H2O( 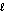 )
)
A) CO32-
B) CO2
C) SO42-
D) H2O
E) H2SO4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Chalk is mostly calcium carbonate. Could chalk-dust
Q8: What volume of 12 M HCl solution
Q10: Sodium fluoride is added to drinking water
Q11: A salt solution is added to a
Q45: The thermite reaction is used in
Q45: A chemistry student attempted to make a
Q54: What is meant by the terms solution,
Q73: Commercial hydrochloric acid is 12.1 M. What
Q84: Magnesium metal burns brightly in the air
Q102: When the oxidation-reduction reaction shown here