Multiple Choice
What would be the products in the ionic equation for the following reaction? 2HBr(aq) + 2KOH(aq) products
I. K+(aq) and Br-(aq) ;
II. KH(aq) ;
III. H2O( 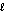 ) ;
) ;
IV. KBr(s) ; V. BrOH(aq) ; VI. KBr(aq)
A) I and III
B) III and IV
C) III and VI
D) II, III, and V
E) II and V
Correct Answer:

Verified
Correct Answer:
Verified
Q13: One type of alcohol breathalyzers used by
Q14: Which one of the following statements regarding
Q15: Which one of the following statements regarding
Q18: In the dilution of 10.0 mL of
Q20: In a titration, the solution of known
Q22: Hydroxyapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH)], the major component of tooth
Q76: The oxidation number of carbon in carbon
Q125: Chlorine, ozone, and chlorine dioxide are all
Q126: A homogeneous mixture of two or more
Q129: If the molar concentration of sodium sulfate