Multiple Choice
Polymers with ionic functional groups have been developed for removal of ions from water. One example is Amberlite. One form of Amberlite has the structure shown below, where the -SO3H groups act like a weak acid. What type of ions will this polymer attract? 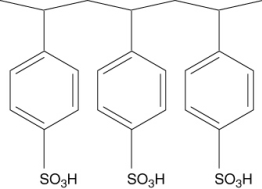
A) cations
B) anions
C) all ions
D) impossible to tell
E) none, since the polymer is neutral
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Why is an aqueous solution of table
Q15: Controlling the ammonia and ammonium ion
Q23: What is the oxidation number of P
Q30: A 500 mg dietary supplement of L-lysine
Q49: The proof of liquor is defined as
Q53: Determine the molar concentration of an aqueous
Q59: In a spontaneous oxidation reduction reaction between
Q62: Sodium thiosulfate (Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>, molar mass = 158.2
Q99: If there are 0.505 g of NaCl
Q100: Sodium metal easily loses an electron to