Multiple Choice
The half-reaction for the reduction of molecular oxygen to water is __________
A) 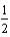 O2(g) H2O(
O2(g) H2O( 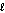 ) .
) .
B) 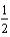 O2(g) + H2(g) H2O(
O2(g) + H2(g) H2O( 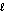 ) .
) .
C) 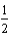 O2(g) + H2(g) + 2e- H2O(
O2(g) + H2(g) + 2e- H2O( 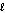 ) .
) .
D) 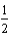 O2(g) + 2 H+(g) + 2e- H2O(
O2(g) + 2 H+(g) + 2e- H2O( 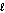 ) .
) .
E) 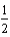 O2(g) + 2 H+(g) H2O(
O2(g) + 2 H+(g) H2O( 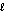 ) + 2e-.
) + 2e-.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: Hydroxyapatite, [Ca<sub>5</sub>(PO<sub>4</sub>)<sub>3</sub>(OH)], the major component of tooth
Q23: What is the molar concentration of sodium
Q27: In which compound does chlorine have an
Q30: Potassium is a very reactive metal. Many
Q37: Ammonia (NH<sub>3</sub>) is a weak base that
Q39: Concentrated sulfuric acid contains 4 g of
Q48: A reaction that takes place between
Q73: How many grams of solid magnesium chloride,
Q76: The oxidation number of carbon in carbon
Q125: Chlorine, ozone, and chlorine dioxide are all