Multiple Choice
Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O ( 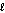 ) 2H2(g) + O2(g)
) 2H2(g) + O2(g)
A) 17.8 g
B) 8.9 g
C) 35.6 g
D) 16.0 g
E) 10.0 g
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many mL of C<sub>16</sub>H<sub>34</sub> are needed
Q8: In a demonstration of the complete combustion
Q9: Identify the set of stoichiometric coefficients that
Q10: Like many metals, manganese reacts with halogens
Q29: Calcium carbide, CaC<sub>2</sub>, reacts with water to
Q68: Arizona was the site of a 400,000-acre
Q110: Which one of the following samples contains
Q119: How many moles of ammonia are there
Q121: What is the molar mass of sulfuric
Q140: Ammonia, NH<sub>3</sub>, is an important industrial chemical.