Multiple Choice
When cosmic rays strike atoms in the upper atmosphere, energetic neutrons are produced. These neutrons collide with nitrogen-14 atoms, producing carbon-14 atoms and hydrogen atoms. Which diagram represents the carbon-14 product? 
A) 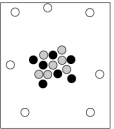
B) 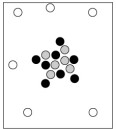
C) 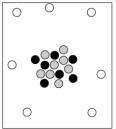
D) 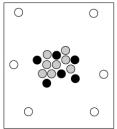
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The average atomic mass of zinc is
Q5: In the atoms in the Rutherford-Geiger-Marsden experiment,
Q6: Which contains more carbon by mass, 1
Q7: Identify the binary compound that has ionic
Q9: What is the correct symbol for an
Q10: Dalton's law of multiple proportions deals with
Q11: Elements 21 through 30 are known as
Q45: The average atomic mass of carbon is
Q96: What is the empirical formula for benzene,
Q137: Give an example of an alkali metal.