Multiple Choice
Write the equation for the production of potassium metal from potassium chloride.
A) KCl(s) + Na(s) 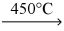 NaCl(s) + K(s)
NaCl(s) + K(s)
B) KCl(s) + Na(s) 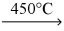 KNaCl(s)
KNaCl(s)
C) KCl(l) + Na(l) 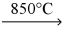 NaCl(l) + K(g)
NaCl(l) + K(g)
D) KCl(l) + Na(g) 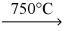 NaCl(s) + K(s)
NaCl(s) + K(s)
E) There is no reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Write an equation for the roasting of
Q17: Choose the INCORRECT statement about Na<sub>2</sub>CO<sub>3</sub>.<br>A) A
Q18: The only by-product obtained in the Solvay
Q19: Finish the following reaction: SnO<sub>2</sub> + NaOH(aq)
Q20: Complete the reaction: SiO<sub>2</sub> + 2 C
Q23: When sodium and potassium react with excess
Q26: What weight of lead sulfate is produced
Q29: Quartz and mica are examples of:<br>A)silanols<br>B)silanes<br>C)silicones<br>D)silicates<br>E)isomers
Q81: What volume of oxygen gas,measured at STP,would
Q111: Choose the INCORRECT statement.<br>A)Sodium chloride may be