Multiple Choice
Choose the INCORRECT statement.
A) The van't Hoff equation is ln 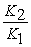 =
=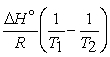 .
.
B) Keq is independent of temperature.
C) In a thermodynamic equilibrium constant expression, the activity of a gas is replaced by its partial pressure in atmosphere.
D) In a Keq expression, the activity of a solution is replaced by its molarity.
E) If ΔG = 0, the process is at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following processes would result
Q35: If ΔG < 0 for a reaction,then
Q36: Choose the INCORRECT statement.<br>A) The third law
Q37: Which material has the largest entropy?<br>A) cannot
Q38: For the reaction, 2 O<sub>3</sub>(g) → 3
Q39: Consider the reaction: AB(g) → A(g) +
Q41: For the reaction, CaCO<sub>3</sub>(s) → CaO(s) +
Q45: What is ΔG° at 25 °C? <br>CaCO<sub>3</sub>(s)
Q87: Which of the following has the highest
Q110: A zero ΔG means the system is