Multiple Choice
Consider the reaction: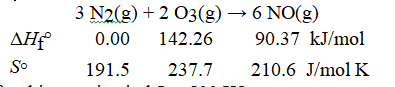
What is ΔG°rxn for this reaction in kJ at 500 K?
A) 93 kJ
B) 151 kJ
C) 365 kJ
D) -1.00 × 105 kJ
E) 441 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Predict whether ΔS is positive or negative
Q4: Which of the following quantities is generally
Q6: Consider the reaction: <br>N<sub>2</sub>O<sub>4</sub>(g) → 2 NO<sub>2</sub>(g)
Q7: For CdO(s) + SO<sub>3</sub>(g) → CdSO<sub>4</sub>(s) ΔH°
Q9: For the reaction, CO(g) + 2H<sub>2</sub>(g) →
Q10: Consider the endothermic reaction: N<sub>2</sub>(g) + O<sub>2</sub>(g)
Q11: Consider the reaction: H<sub>2</sub>X(g) → HX(g) +
Q12: What is ΔG°rxn? <br>CO(g) + 2 H<sub>2</sub>(g)
Q13: Indicate the statement(s) which are true for
Q36: A spontaneous process:<br>A)will happen quickly<br>B)releases large amounts