Multiple Choice
Calculate ΔG° for the reaction Cu(s) + H2O(g) → CuO(s) + H2(g) at 500K. 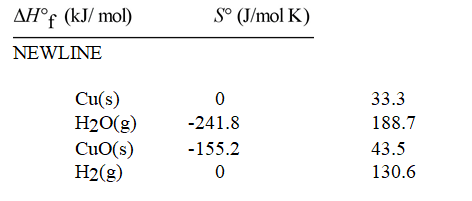
A) 231.8 kJ
B) -135.4 kJ
C) -58.6 kJ
D) 110.6 kJ
E) 86.74 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Choose the INCORRECT statement about coupled reactions.<br>A)The
Q58: The change in Gibbs energy of a
Q91: For the reaction I<sub>2</sub>(s) + Cl<sub>2</sub>(g) →
Q92: The maximum quantity of energy available for
Q93: Consider the following reaction: <br>4 NH<sub>3</sub>(g) +
Q94: For the reaction PCl<sub>5</sub>(g) → PCl<sub>3</sub>(g) +
Q95: Predict whether ΔS is positive or negative
Q96: Consider the following reaction:<br> 4 NH<sub>3</sub>(g) +
Q97: The following reaction is endothermic. 2NH<sub>3</sub>(g) →
Q98: The entropy of a system always increases