Multiple Choice
Consider the reaction: 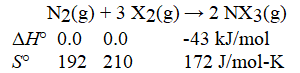
What is ΔG° for this reaction at 591 K? Is the reaction spontaneous at 591 K?
A) -196 kJ/mol, no
B) 196 kJ/mol, yes
C) 196 kJ/mol, no
D) 239 kJ/mol, yes
E) -239 kJ/mol, no
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Standard Gibbs energy of formation requires the
Q26: Entropy is related to the way in
Q57: What is ΔG° at 25°C? <br>2O<sub>3</sub>(g) →
Q57: Choose the INCORRECT statement.<br>A)One form of the
Q58: If the enthalpy of vaporization of chloromethane,
Q60: For a reaction Keq = 1.2 ×
Q62: What is ΔG°rxn? <br>2 O<sub>3</sub>(g) → 3
Q63: For the reaction SO<sub>2</sub>(g) + Cl<sub>2</sub>(g) →
Q64: Calculate the temperature for which Keq for
Q85: A reaction is spontaneous if:<br>I.ΔG is a