Multiple Choice
When equal volumes of the indicated solutions are mixed, precipitation should occur only for: 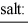
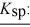 barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
barium fluoride 1.0 × 10-6 calcium carbonate 2.8 × 10-9
Calcium fluoride 5.3 × 10-9
Magnesium fluoride 3.7 × 10-8
Silver carbonate 8.5 × 10-12
A) 2 × 10-5 M Ag+ + 2 × 10-5 M CO32-
B) 2 × 10-5 M Ca2+ + 2 × 10-5 M CO32-
C) 2 × 10-5 M Ca2+ + 2 × 10-3 M F-
D) 2 × 10-2 M Mg2+ + 2 × 10-3 M F-
E) 2 × 10-3 M Ba2+ + 2 × 10-3 M F-
Correct Answer:

Verified
Correct Answer:
Verified
Q5: In which of the following one molar
Q6: In a qualitative cation analysis, the unknown
Q7: The solubility product constant of PbI2 is
Q8: If chromium(III) chloride is added to be
Q9: Write the solubility product constant expression for
Q11: Saturated solutions of sodium sulfate, ammonium carbonate,
Q12: Concentrated solutions of iron (III) chloride, sodium
Q13: What is the free Ag<sup>+</sup> concentration of
Q14: In a qualitative cation analysis, the unknown
Q15: The molar solubility of SrSO<sub>4</sub> (Ksp =