Multiple Choice
The titration curve for 10.0 mL of 0.100 M H3PO4(aq) with 0.100 M NaOH(aq) is given below. 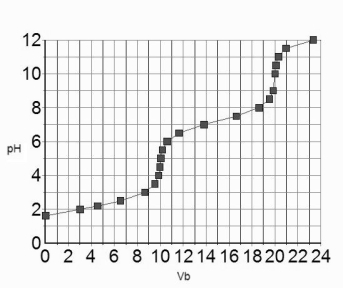 Estimate the pKa2 of H3PO4.
Estimate the pKa2 of H3PO4.
A) 7.2
B) 4.8
C) 9.8
D) 2.2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: What volume in ml of 0.05 M
Q56: For the following titration, determine whether the
Q57: Choose the correct statement.<br>A) 30 mL of
Q59: A solution of sodium carbonate is easier
Q61: Phenol red indicator changes from yellow to
Q62: Assuming no volume change on mixing, what
Q63: Ten milliliters of 0.10 M NH<sub>3</sub>(aq) (K
Q64: What is the buffer range (for an
Q65: A solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100 M
Q103: Phenolphthalein may be used as an indicator