Multiple Choice
According to the phase diagram given, which of the following statements is INCORRECT? 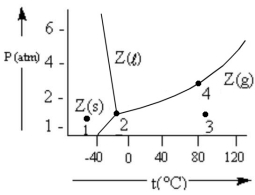
A) At the temperature and pressure of point 2, substance Z exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 3, substance Z exists as a one-phase gaseous system.
C) If the Z(s) = Z(l) = Z(g) system is maintained at the temperature of point 2 while pressure is decreased, more Z will vaporize.
D) If liquid Z is maintained at the pressure of point 4 while the temperature is decreased to 30°C, the liquid will vaporize.
E) The existence of liquid Z at -50°C and 2 atm represents the metastable condition of "supercooling."
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The temperature at which the vapor pressure
Q3: Arrange the following compounds in order of
Q3: According to the phase diagram given, which
Q4: Which of the substances below would produce
Q5: Coordination number is:<br>A) The number of atoms
Q8: Van der Waals forces are a type
Q10: What is the maximum number of glycerol
Q12: Which of the following compounds is the
Q60: Which of the following ionic compounds should
Q87: The property of a liquid that measures