Multiple Choice
According to the phase diagram given, which of the following statements is wrong? 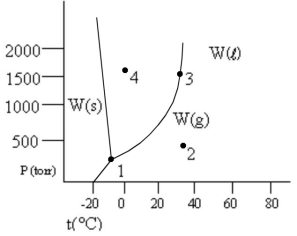
A) At the temperature and pressure of point 1, substance W exists as a three-phase equilibrium system.
B) At the temperature of point 2, a pressure of 500 torr is sufficient to liquify gaseous W.
C) If the W(l) = W(g) system is maintained at the temperature of point 3 while pressure is decreased, more W will vaporize.
D) If liquid W is maintained at the pressure of point 4 while the temperature is increased to 80°C, the liquid will vaporize.
E) The existence of liquid W at -40°C and 500 torr represents the metastable condition of "supercooling."
Correct Answer:

Verified
Correct Answer:
Verified
Q39: There are no types of crystalline solids
Q61: The factor that has the largest effect
Q62: Find a FALSE statement about X-rays.<br>A) diffracted
Q64: Below are given the Lewis structures of
Q67: Which of the following compounds has the
Q68: What is the difference between "normal boiling
Q69: A crystal does not conduct electricity, even
Q70: A crystal does not conduct electricity, yet
Q71: The enthalpy of fusion is:<br>A) The quantity
Q80: The maximum temperature at which a gas