Multiple Choice
Given the following information, calculate ΔH°(in kcal mol-1) for:
NaI(s) → Na+(g) + I-(g) 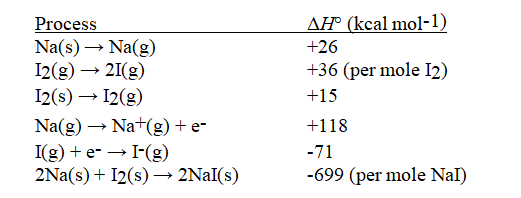
A) 816 kcal mol-1
B) 823 kcal mol-1
C) 562 kcal mol-1
D) 746 kcal mol-1
E) 798 kcal mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The temperature at which the vapor pressure
Q8: Van der Waals forces are a type
Q10: What is the maximum number of glycerol
Q12: Which of the following compounds is the
Q15: Given the data below, determine the normal
Q16: Which probably has the lowest boiling point
Q26: When the vapor pressure of a liquid
Q31: Under which of the following conditions will
Q47: Surface tension is thought to be due
Q60: Which of the following ionic compounds should