Multiple Choice
For the molecule 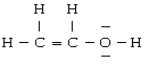
A) the geometry about O is linear
B) the hybridization on O is sp
C) O is not hybridized
D) both carbons are sp2 hybridized
E) there are two π bonds between the two carbons
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Which of the following carbon molecules has
Q27: The double covalent bond between two carbon
Q29: Which of the following could act as
Q30: How many π-electrons are there in S<sub>2</sub>O
Q32: Which of the following involves delocalized π
Q33: If the HCOO- ion is described using
Q34: For XeF4, what are the dipole moment
Q35: The HCOO- ion can be described by
Q59: Choose the INCORRECT statement about HCN.<br>A)There are
Q94: A triple bond is two sigma bonds