Multiple Choice
Which statement is INCORRECT about molecular orbital theory?
A) The number of molecular orbitals produced is equal to the number of atomic orbitals combined.
B) Each pair of sigma molecular orbitals is a bonding orbital and an antibonding orbital.
C) The antibonding orbital is at a lower energy than the bonding orbital.
D) The bond order (BO) is 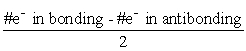 .
.
E) Hund's rule says that each orbital of identical energy has one electron before pairs are formed.
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which of the following statements concerning molecular
Q73: Unhybridized p orbitals must be present for
Q81: What is the hybridization of the S
Q83: Choose the INCORRECT statement about SnCl<sub>2</sub>.<br>A) The
Q84: Choose the INCORRECT statement about PCl5.<br>A) There
Q85: According to M.O. theory, which is an
Q87: According to MO theory, assuming that the
Q88: Which of the following statements concerning the
Q89: The electrical conductivity of semiconductors increases with
Q91: According to the band theory, why is