Multiple Choice
One resonance structure of N2O is 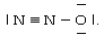 The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
The hybridized atomic orbitals of the central nitrogen atom which are consistent with this structure are ________.
A) four sp3 orbitals
B) three sp2 orbitals and a p orbital
C) two sp2 orbitals and two sp orbitals
D) two sp orbitals and two p orbitals
E) one sp orbital and two p orbitals
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Which statement is correct for the structure
Q67: The inclusion of a small amount of
Q70: According to the valence bond theory, in
Q72: The geometry of sp2 hybridized orbitals is
Q73: The hybridization on Xe in XeF4 is
Q74: How many σ- and π-bonds, respectively, are
Q75: Which of the following statements concerning the
Q76: According to the principles of VSEPR applied
Q81: If the wave functions describing the 2s
Q89: The concept of an anti-bonding orbital is