Multiple Choice
Write a Lewis structure for barium fluoride.
A) 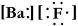
B) F-B-F
C) 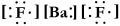
D) 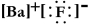
E) 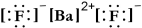
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: In a Lewis structure,a terminal atom is
Q73: What is the formal charge on N
Q74: Choose the correct statement about the compound
Q75: Compound EX4 reacts with Y2 giving two
Q76: Write a Lewis structure for BaO.<br>A) <img
Q78: Given the bond enthalpies I-Cl (209), H-H
Q79: Which of the following has a molecular
Q80: Which compound would be expected to have
Q81: Which of the following exhibits ionic bonding?<br>A)
Q82: Given the bond enthalpies C-O (360), C=O