Multiple Choice
A cation has 13 neutrons and 10 electrons. If it has a charge of +1, what is its correct symbol?
A) 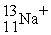
B) 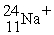
C) 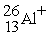
D) 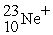
E) 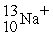
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: A 0.920-gram sample of magnesium is allowed
Q55: A 3.214 g sample of magnesium reacts
Q66: An element has 5 stable isotopes. The
Q68: Magnesium has 3 stable isotopes with masses
Q71: The total numbers of neutrons, protons, and
Q73: Lead has 4 stable isotopes with masses
Q74: The natural abundance of calcium in the
Q75: How many moles of Fe is present
Q96: Sodium is a member of the family
Q114: What mass of magnesium is necessary to