Multiple Choice
An anion has 45 neutrons and 36 electrons. If it has a -1 charge, what is its correct symbol?
A) 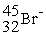
B) 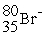
C) 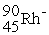
D) 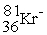
E) 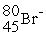
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: There are two stable isotopes of supposium
Q46: The total numbers of neutrons, protons, and
Q47: The total number of neutrons in an
Q50: How many moles are represented by 2.5
Q54: Write the appropriate symbol for the species
Q55: What is the atomic weight of an
Q56: Which of the following statements is FALSE?<br>A)
Q63: What is the mass of a sample
Q86: Choose the correct statement.<br>A)Neutrons have no charge
Q93: Choose the INCORRECT statement.<br>A)Gamma rays are bent