Multiple Choice
The average atomic mass of B is 10.80 u. Boron has only two stable forms 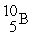 (10.00 u) and
(10.00 u) and 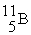 (11.00 u) . What is the natural percent abundance of
(11.00 u) . What is the natural percent abundance of 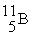 ?
?
A) 80%
B) 20%
C) 1.0%
D) 3.8%
E) 75%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: When a chemical reaction is carried out
Q20: A hypothetical element,E,has two stable isotopes:<br>E-46 =
Q39: What is the mass number of the
Q69: Choose the information a mass spectrometer is
Q75: J.J.Thomson suggested the "plum pudding" model of
Q77: 57.7 g Ni contains how many atoms?<br>A)
Q78: Choose the INCORRECT statement.<br>A) The Law of
Q82: A sample of pure carbon weighing 1.48
Q84: An element has 5 stable isotopes. The
Q99: All matter is composed of atoms.