Multiple Choice
Which of the following is the correct "setup" for the problem "How many grams of H2O will be produced from 2.1 moles of O2 and an excess of H2S?" according to the reaction 2H2S + 3O2  2H2O + 2SO2
2H2O + 2SO2
A) 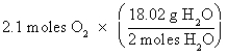
B) 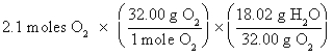
C) 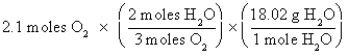
D) 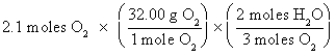
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Select the set of coefficients from the
Q3: To determine the formula mass of a
Q4: Which set of coefficients balances the equation
Q5: Characterize EACH of the three given statements
Q6: In which of the following unbalanced equations
Q7: Characterize EACH of the three given statements
Q8: One mole of H<sub>2</sub>SO<sub>4</sub> contains<br>A) two moles
Q9: Which of following statements concerning theoretical yield,
Q10: Select the correct numerical value for the
Q11: A compound with the formula TeCl<sub>n</sub> has